Vaccine data for children under five by April: Pfizer
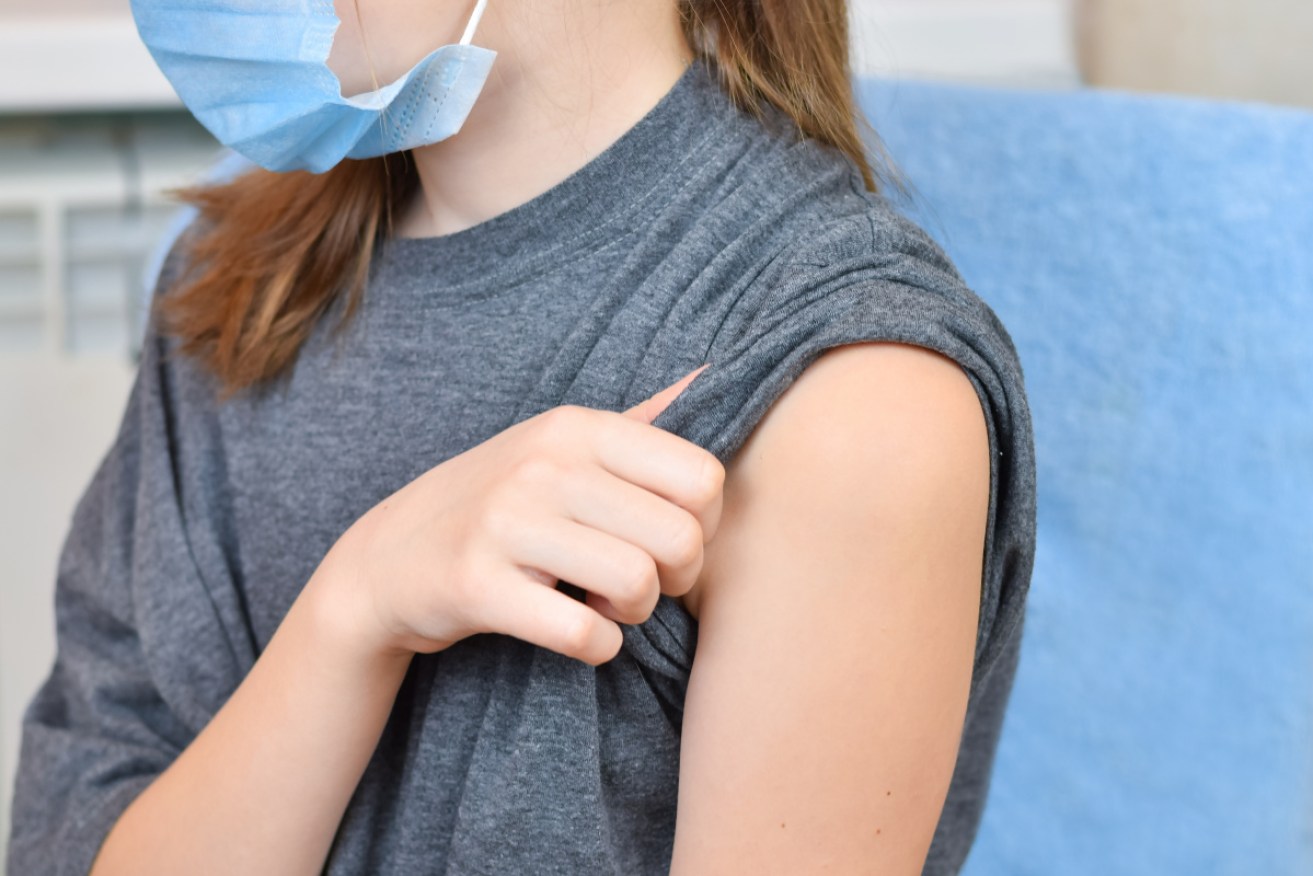
The Australian Technical Advisory Group on Immunisation has recommended extending eligibility for the Pfizer booster to about 120,00 children starting on June 14. Photo: Getty
Pfizer expects the latest results from a clinical trial of its COVID-19 vaccine for children under the age of five by April, a leading company scientist says.
“The study has been amended to give a third dose to everybody who’s less than five at least eight weeks after their last vaccination,” Dr Alejandra Gurtman, a Pfizer vaccine researcher, said at a meeting of the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
She said the company aims to have data for the age group by the end of March or beginning of April.
In December, Pfizer said it was changing the design of the trial because children between the ages of two and four who were given two three-microgram doses of the vaccine did not have the same immune response that a larger dose of the vaccine generated in older children.
Dr Gurtman also said the company was studying a third dose of its vaccine in children ages five to 11, six months after their second dose.
On December 5, 2021, the Therapeutic Goods Administration provisionally approved the Pfizer vaccine for use among five to 11-year-old children in Australia from January 10.








