‘Hundreds of thousands’ Chinese get experimental COVID vaccine

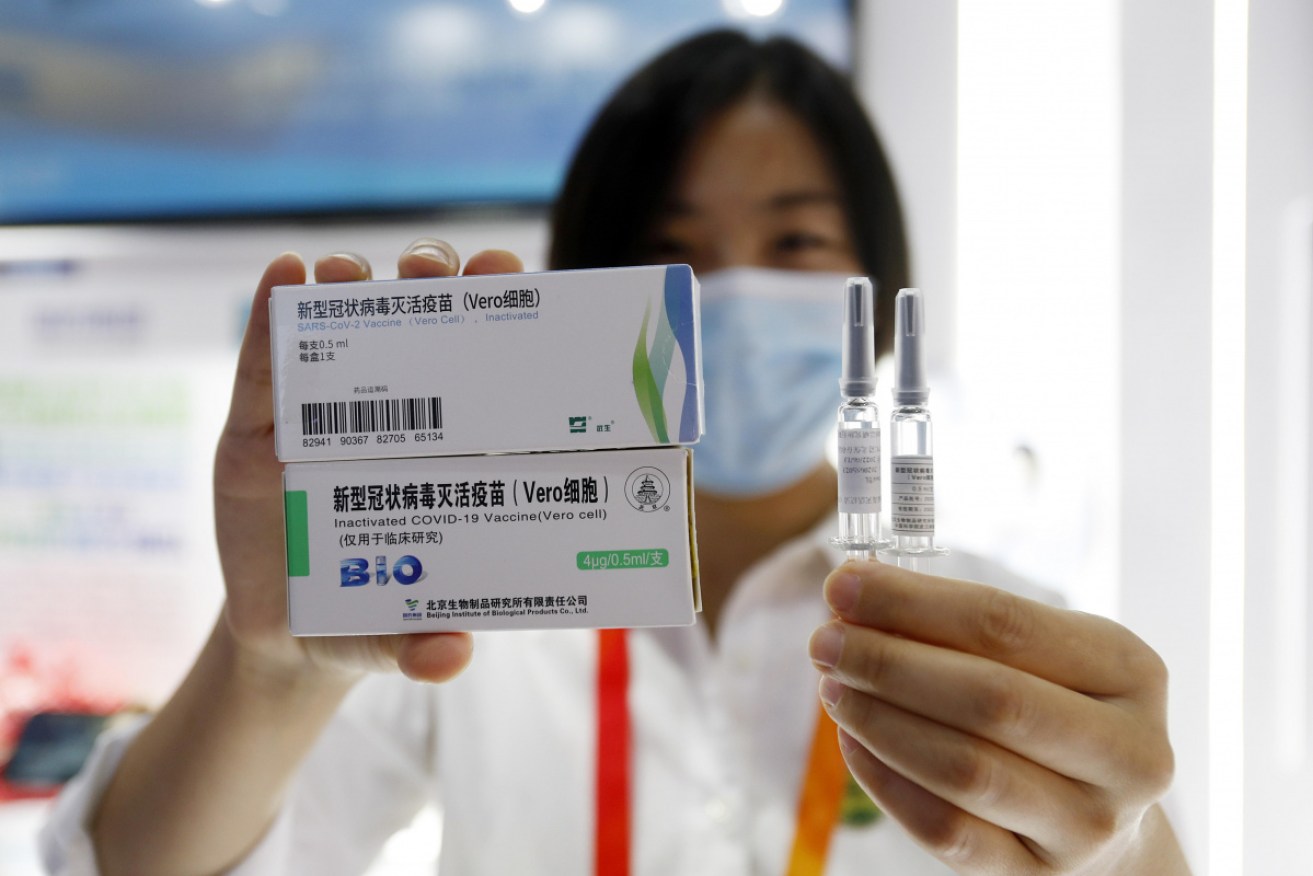
Inactivated COVID-19 vaccine candidate from CNBG at the International Fair for Trade in Services, Beijing Olympic Park Photo: Getty
Tens of thousands … even hundreds of thousands of Chinese citizens have been given two Chinese COVID-19 vaccine candidates as part of an emergency-use program.
Not one of them has become sick with the coronavirus. And not one of them has suffered an adverse effect. The emergency program (said to have started in July) is only six weeks old so… wow to all that.
These are the widely reported claims in wire-service stories of a senior official of a Chinese state-owned vaccine developer – and they have spawned plenty concerns about the program’s safety.
The vaccines have not gone through rigorous clinical trials. Emergency-use means the vaccines are being deployed with the assumption they work, and no doubt with many fingers crossed.
Could it be true? Who knows?
The claims come in the same week that Donald Trump is reportedly at loggerheads with his health officials over when a COVID-19 vaccine will be safely and effectively delivered to the American people.
Mr Trump says an effective vaccine will be ready by next month, just ahead of the US election.
The head of the US Centre of Disease Control has said that a vaccine might be trialled on a small number of Americans before the end of the year, but won’t go into a significant number of arms until mid-2021, at the earliest.
The Chinese claims also come as the much-vaunted Oxford trial recovers from a stumble in the form of a severe reaction in one of their Phase III participants.
What the Chinese are claiming
According to AAP, the vaccine emergency-use program that offers three experimental vaccines to essential workers including medical, transport and food market workers.
The vaccines are being developed by state pharmaceutical giant China National Pharmaceutical Group (Sinopharm) and US-listed Sinovac Biotech.
In June, a fourth vaccine being developed by CanSino Biologics was approved for use by the Chinese military.
Beijing has not released official data on the uptake in domestic targeted groups.
But China National Biotec Group (CNBG) — the Sinopharm unit developing two of the emergency-use vaccines — and Sinovac have confirmed that at least tens of thousands of people have been inoculated.
CNBG also said it had given hundreds of thousands of doses, as one of its vaccines requires two or three shots for an individual to be inoculated.
Beijing has engaged a public, top-down approach to endorse the experimental vaccines and foster community support.
Among those lining up for vaccinations early on were the chief executives of Sinovac and Sinopharm and the military’s research chief.
Dr Guizhen Wu, the chief bio-safety expert for China’s National Institute for Viral Disease Control and Prevention, said on state TV this week that she had received a vaccination in April.
April! That is six months ago
“So far, among the people who were vaccinated, no one has been sick with the disease. So far, [the vaccination scheme] works very well. No side effect occurred,” said Dr Wu, who believes some of the vaccines would be ready for public use as early as November.
Dr Wu’s comments were broadly in line with comments by CNBG last week that none of tens of thousands of people who travelled to high-risk countries and regions after being vaccinated had been infected and there was “no case of obvious adverse reaction”.
But how would they know? Hundreds of thousands of people have been jabbed in six weeks, according to the Wall Street Journal. In emergency-use conditions no one is being closely monitored, as they would be in a clinical trial setting. Which means in a hospital bed.
Russia and India back the wild-card method
China’s approach runs counter to that of many Western countries, where experts have warned against authorising the emergency use of vaccines that have not completed testing, citing a lack of understanding about longer-term efficacy and potential side effects.
Russia is one of the few other countries to authorise the use of an experimental vaccine, making its own ‘Sputnik V’ vaccine mandatory for certain groups, including teachers.
India is considering emergency authorisation for a vaccine, particularly for the elderly and people in high-risk workplaces.
– with AAP