China approves coronavirus drug for wide use: ‘Clearly effective’

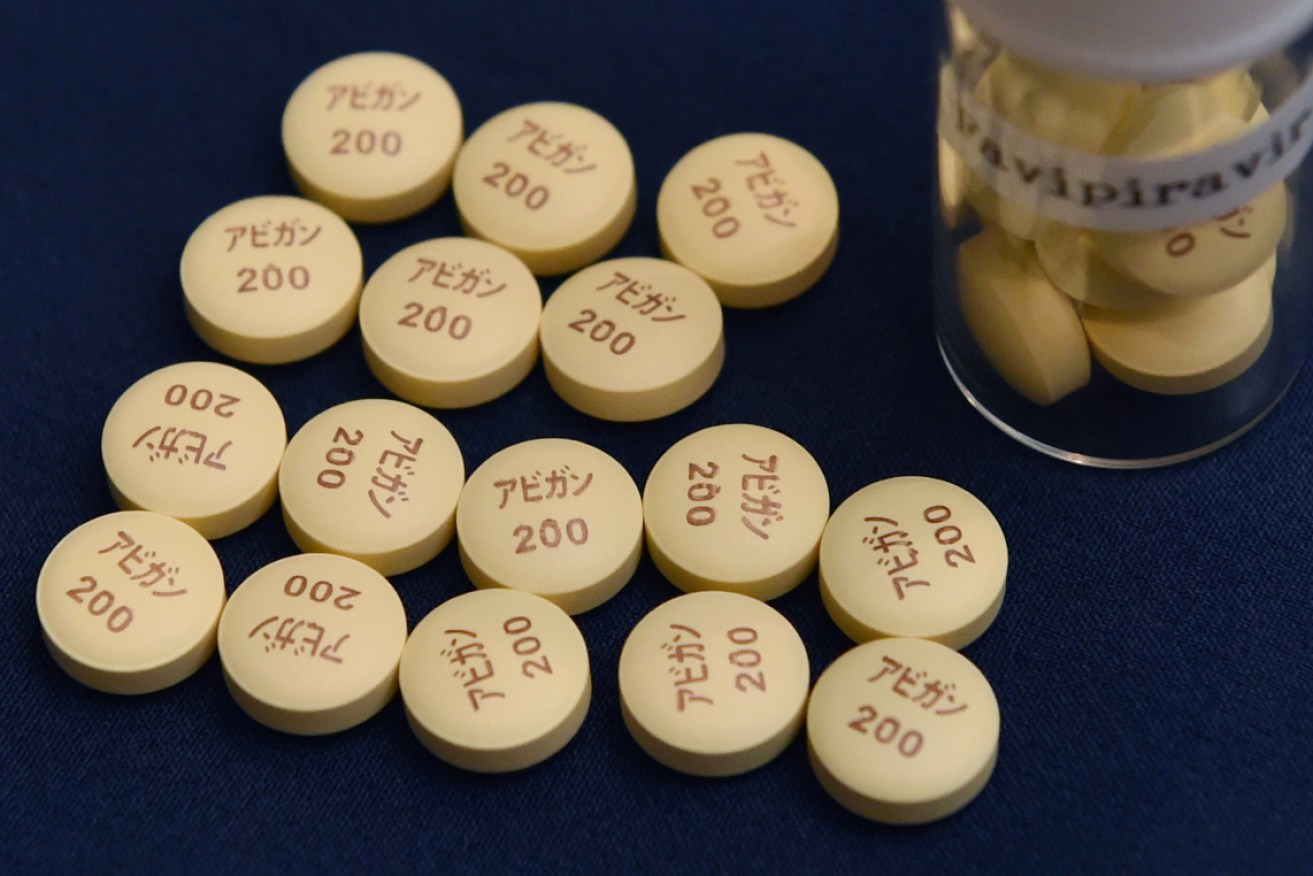
A Japanese company developed the drug favipiravir in 2014 to combat seasonal flu. Photo: Getty
It’s being widely reported that a Japanese flu drug is “clearly effective” against the coronavirus, at least in less severe cases. China has officially advised the use of the anti-viral drug by anyone sick with the Covid-19.
Here’s what we know from multiple reports:
- The drug, favipiravir, is sold under the brand name Avigan, and was developed by Fujifilm Toyama Chemical in 2014.
- About 340 patients in the Chinese cities of Wuhan and Shenzen received the drug as part of clinical trials. The New Daily has not seen the trial’s data or a journal paper.
- Patients tested negative for the virus in an average of four days, instead of the 11 days in the control group, according to Zhang Xinmin, director of the Chinese government’s National Center for Biotechnology Development. “It is very safe and clearly effective,” he said in a news conference.
- He said that it took an average of 2.5 days for the temperature of those who were given Avigan to return to normal. It took 4.2 days for those who weren’t given the drug.
- He said that patients given the medicine were able to get rid of their cough in an average of 4.57 days, compared with 5.98 days for those who were not given it. He added that the medicine had no obvious side effects.
- The trial reportedly also found that X-ray photos confirmed improvements in lung conditions in about 91 percent of the patients who were given the medicine. The number stood at 62 percent for those who weren’t given it.
- Only 8.2 cent of the patients taking favipiravir needed respiratory aids, whereas 17.1 per cent of the patients in the control group were put on devices.
- Shares of Fujifilm spiked more than 15 per cent Wednesday in response.
- Japanese doctors are also testing favipiravir in clinical trials, but the drug was not as effective in patients with more severe symptoms, The Guardian reported, citing the country’s health minister.
- A Fujifilm spokesperson told Nikkei that the company is not involved in the Chinese clinical trials and is currently evaluating them. However, Japan is doing its own trails with the drug.
- A Japanese health ministry was quoted in the newspaper the Manichi Shimbun that the drug so far has been given to around 70 to 80 people, but that early results suggest it isn’t effective in treating those with more severe symptoms where the virus has already multiplied to a much greater extent.
- It’s been reported that Fujifilm is also supplying the drug to South Korea for testing. Other reports suggest South Korea resisted importing the drug..
- While the Chinese government has said there are no side effects, some reports cited unnamed studies that found the drug can cause foetal deformities and deaths.
- According to the Nikkei Asian Review, the drug obtained regulatory approval in 2014 on condition that it would only be used if the government decided to fight new or re-emerging influenza viruses.
- Other drugs that have shown early promising signs include remadesivir, a compound developed by Gilead Sciences that has shown some promise as a general antiviral. But favipiravir is the first drug to get state approval as a coronavirus treatment.
The Therapeutic Goods Administration, responding to questions, replying by email said, ” The TGA is aware of and monitoring clinical trials relating to the treatment of Covid-19 for all therapeutic goods, including various clinical trials being conducted internationally that involve the drug favipiravir.
“Various medicines are being trialled, of which favipiravir is but one. There have been no publications in major medical journals of the efficacy of favipiravir at this stage.
“No clinical trial notifications or applications for favipiravir have been received by the TGA to this time.
“Trials regarding this and other products are still in preliminary phases and results should be submitted and evaluated before being assessed as an effective treatment for Covid-19.”
We will update this story as new developments come to hand.